CANCERPLEX delivers actionable and optimal clinical treatment decisions for patients 93% of the time1.

CANCERPLEX SPECIFICATIONS

Genes Analyzed

Clinical Actionability

Sensitivity & Specificity
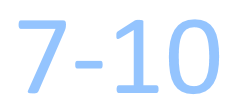
Days Turnaround Time

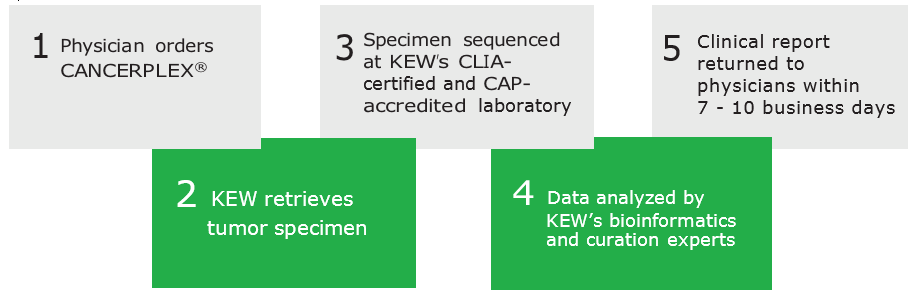
THE CANCERPLEX PROCESS
KEW is actively seeking molecular lab partners. If you want CANCERPLEX be performed by your local pathology lab, please contact us.

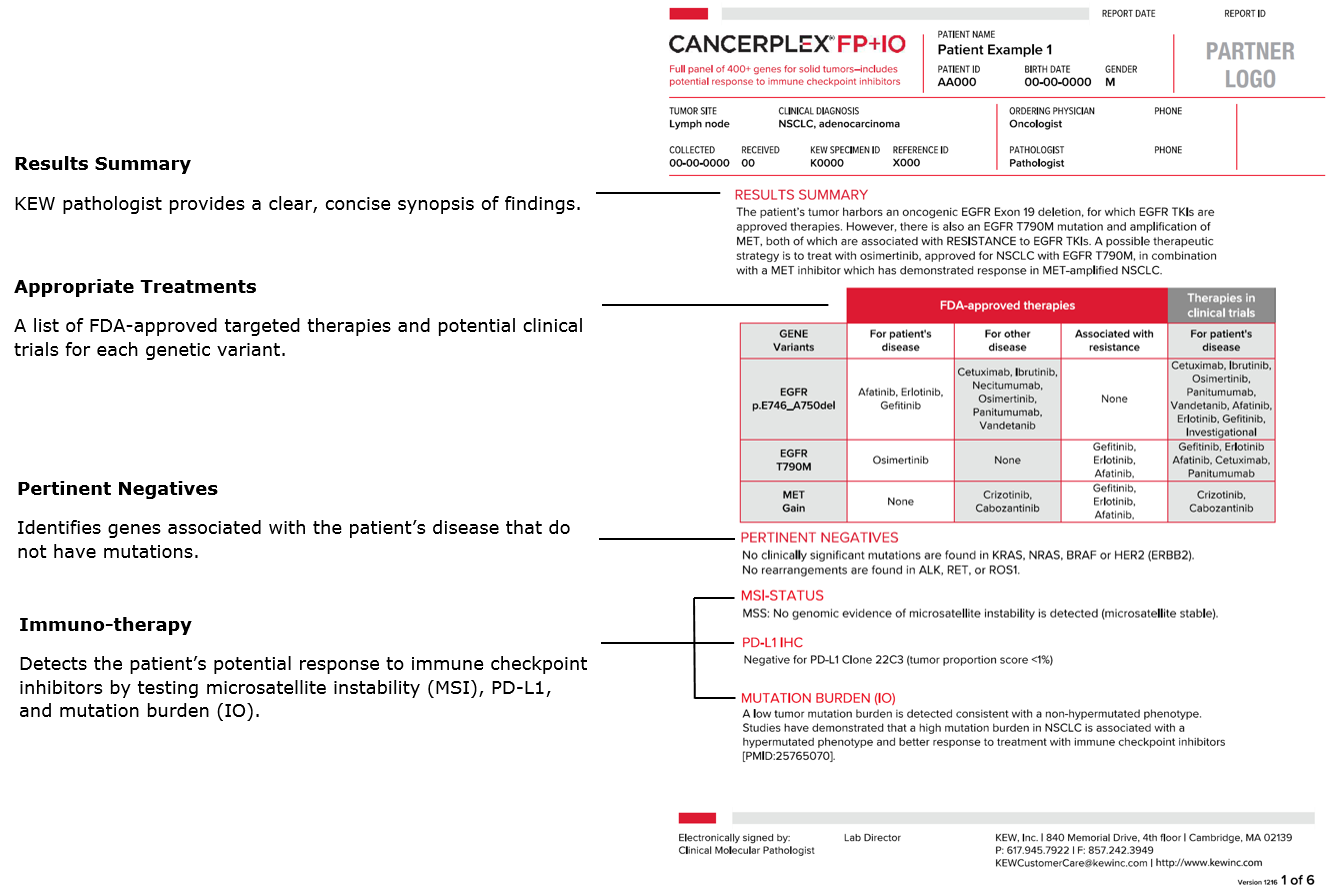
CANCERPLEX REPORT
The clinical report averages 3 − 5 pages with the most important information summarized on the first page. Choose either a full panel or a specialized panel so the report can highlight the level of information you need.

If you need more information, please call KEW Customer Care at 855.225.4068.
Frequently Asked Questions
What patients are eligible for a CANCERPLEX test?
Any patient with a malignant solid tumor is eligible for a CANCERPLEX test.
How do I order a CANCERPLEX test?
You must be a physician to order CANCERPLEX. To order a test, follow the steps described in the ORDER CANCERPLEX section above.
What are the specimen requirements for a CANCERPLEX test?
Download our specimen requirements here.
How many genetic alterations does CANCERPLEX test for?
It depends on the specific test ordered. The CANCERPLEX FP (Full Panel) sequences 400+ genes known to be associated with cancers. Click here for detailed CANCERPLEX information.
Can I request that KEW contact our pathology laboratory directly to obtain the tissue sample?
Yes. When ordering CANCERPLEX, you should fill out the requisition form and include information about the pathology laboratory that holds the sample. Then KEW will contact the laboratory to retrieve the sample.
How long will it take to receive the results report?
The test results will be emailed or faxed to you within 7 – 10 business days after KEW receives the tissue sample from the pathology laboratory.
What information is contained in the results report?
The simple, easy to understand CANCERPLEX report accounts for all current knowledge around targeted oncology treatments. Each report provides the list of genomic alterations within the solid tumor tested and provides a list of relevant FDA-approved targeted therapies or targeted therapies in clinical trials specific to the patient’s specimen. Download a sample report
Is the CANCERPLEX test covered by insurance?
Is the CANCERPLEX test covered by Medicare?
KEW accepts 100% of the Medicare allowable amount for our services. If there is a deductible, co-insurance or co-payment, the patient will be responsible for those amounts.
If the patient has a supplemental insurance to Medicare, we will also bill this secondary insurer as long as the information for the insurer has been provided.
What if my patient doesn’t have insurance or wants to pay directly?
If your patient does not have insurance, or wishes to self-pay, please have them call 855.225.4068 and press 1.
What is the “14 Day Rule”?
The “14 Day Rule” (Medicare Date of Service Regulation 42 C.F.R §414.510) outlines whether or not a laboratory, such as KEW, can bill Medicare for clinical laboratory services based on when a Medicare patient was discharged, including outpatient discharge.
The “14 Day Rule” is triggered by the date the testing was ordered by the physician. If a physician submits an order less than 14 days after an inpatient or outpatient is discharged, KEW will bill the hospital for performing the test. If a physician submits an order more than 14 days after the inpatient or outpatient is discharged, KEW will bill Medicare.

- Eifert C, Pantazi A , Sun R, Xu J, Cingolani P, Heyer J, Russell M, Lvova M, Ring J, Tse J, Lyle S, Protopopov A. Clinical application of a cancer genomic profiling assay to guide precision medicine decisions. PERSONALIZED MEDICINE. Vol.14,NO.4
Call us
855.225.4068Location
303 Wyman Street, Office #349
Waltham, MA 02451
