
CANCERPLEX® is a large-gene panel consisting of >400 cancer-associated genes for the detection of genetic abnormalities underlying cancer with 95->99% sensitivity and specificity at low allelic fractions, determinations of Microsatellite Instability (MSI-H) and Tumor Mutation Burden (TMB-H) status to identify patients who are most likely to respond to immune checkpoint inhibition, and for the presence of HPV/EBV viral integration which may impact treatment decisions. All data generated from sequencing at the local laboratory are processed through our high-quality, high-performance analytics platform, which combines state-of-the-art, industry-leading bioinformatics tools for variant detection with GENEKEEPER® , our proprietary software and knowledgebase for variant interpretation. To date, we have profiled thousands of patients with CANCERPLEX and are proud to have reported clinically actionable variants in 93% of these patients1.
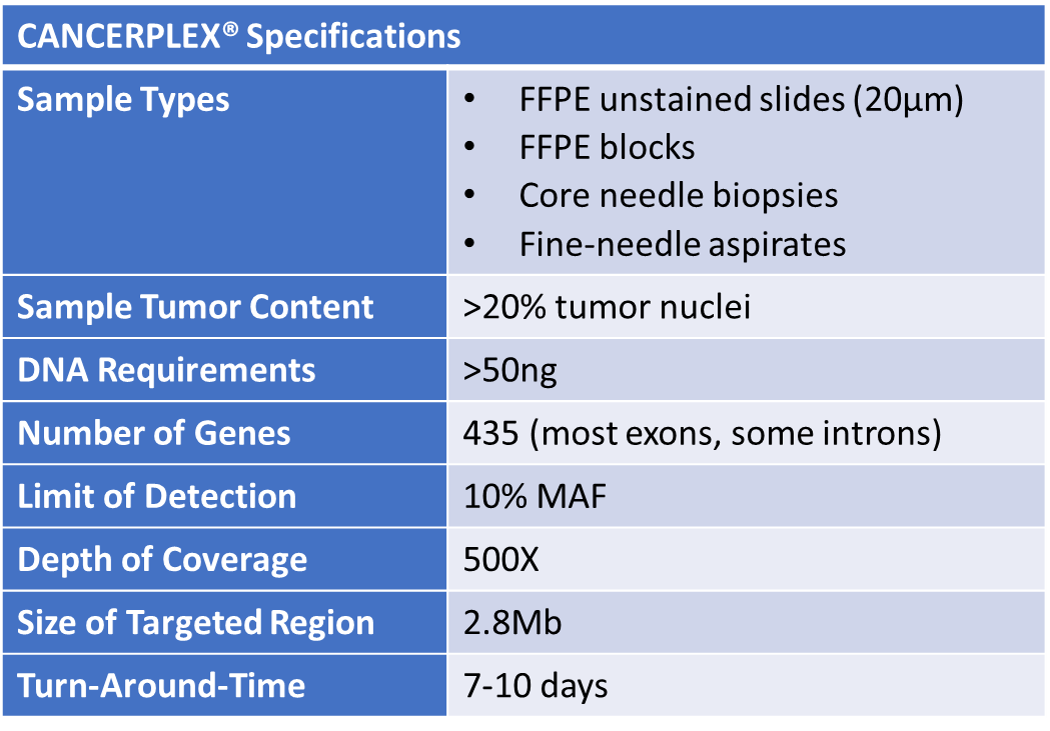

CANCERPLEX WORKFLOW

CANCERPLEX COVERS
FDA-Approved & Investigational Drug Targets
Microsatellite Instability & Tumor Mutation Burden
Therapeutic Targetable Pathways
Drug Resistance Genes

CANCERPLEX ANALYTICAL PERFORMANCE
|
|
Sensitivity |
Specificity |
|
Single Nucleotide Variants |
99.5% |
>99% |
|
Insertions and Deletions |
>99% |
>99% |
|
Copy Number Variation gain (>2.5 fold change) |
98.1% |
>99% |
|
Copy Number Variation loss (<0.75 fold change) |
>99% |
>99% |
|
Structural Variants |
>99% |
>99% |
|
Microsatellite Instability |
>99% |
>99% |
|
Viral Integration |
>99% |
>99% |
*10% MAF detection limitation

CANCERPLEX REPORT
The report contains a list of potential therapies targeting the genomic changes unique to the patient, including
FDA-approved therapies in patient’s cancer type
Clinical trials enrolling patients with the cancer type
FDA-approved therapies in other cancer types

CANCERPLEX ADVANTAGES

High success rate
Quality results obtained from as little as 50ng DNA in >94% tissue samples
Fast turnaround time
Only 7-10 business days

Comprehensive molecular profile
- All types of possible genomic changes (e.g., SNVs, indels, CNVs and fusions)
- The patient’s likelihood to respond to immunotherapy (i.e., tumor mutational burden and MSI)
- Cancer-related viruses

Variety of tissue types
◊ Solid tumors ◊ Pleural effusions ◊ Fine needle aspirates
Unparalleled clinical actionability
93% of patients matched with targeted therapies
Dedicated real-time support
- KEW updates its gene panels and GENEKEEPER database to stay current and actionable
- KEW provides one-on-one support to oncologists to help make the process go smoothly and to answer any questions they may have about the test results

Learn more about how comprehensive genomic profiling can benefit you.


- Eifert C, Pantazi A , Sun R, Xu J, Cingolani P, Heyer J, Russell M, Lvova M, Ring J, Tse J, Lyle S, Protopopov A. Clinical application of a cancer genomic profiling assay to guide precision medicine decisions. PERSONALIZED MEDICINE. Vol.14,NO.4
Call us
855.225.4068Location
303 Wyman Street, Office #349
Waltham, MA 02451
