
CANCERPLEX FOR IMMUNO-ONCOLOGY
In May 2017, the U.S. Food and Drug Administration granted accelerated approval to pembrolizumab (Keytruda®), an immune checkpoint inhibitor for PD-1, for adult and pediatric patients with unresectable or metastatic solid tumors that have been identified as having Microsatellite Instability (MSI-H) or Mismatch Repair Deficiency (dMMR).
This is the first time the FDA has approved a cancer treatment based on a common biomarker rather than the anatomical location where the tumor originated, a pivotal milestone for cancer patients.
CANCERPLEX identifies patients who are MSI-H as candidates for Keytruda and other immune checkpoint inhibitors.
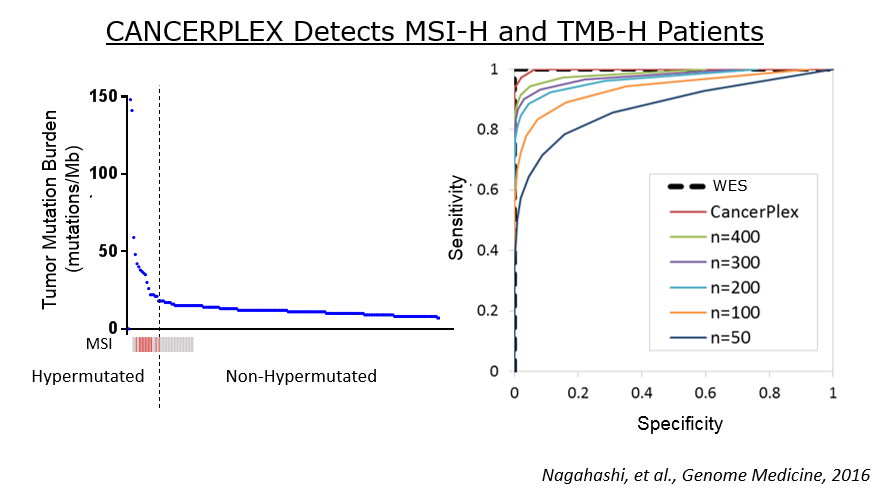
A panel of at least 400 genes is required to accurately determine the tumor mutation burden, which makes our panel of 435 genes an optimal test for targeting the right patients most likely to respond to immuno-oncology therapy.
Call us
855.225.4068Location
303 Wyman Street, Office #349
Waltham, MA 02451
